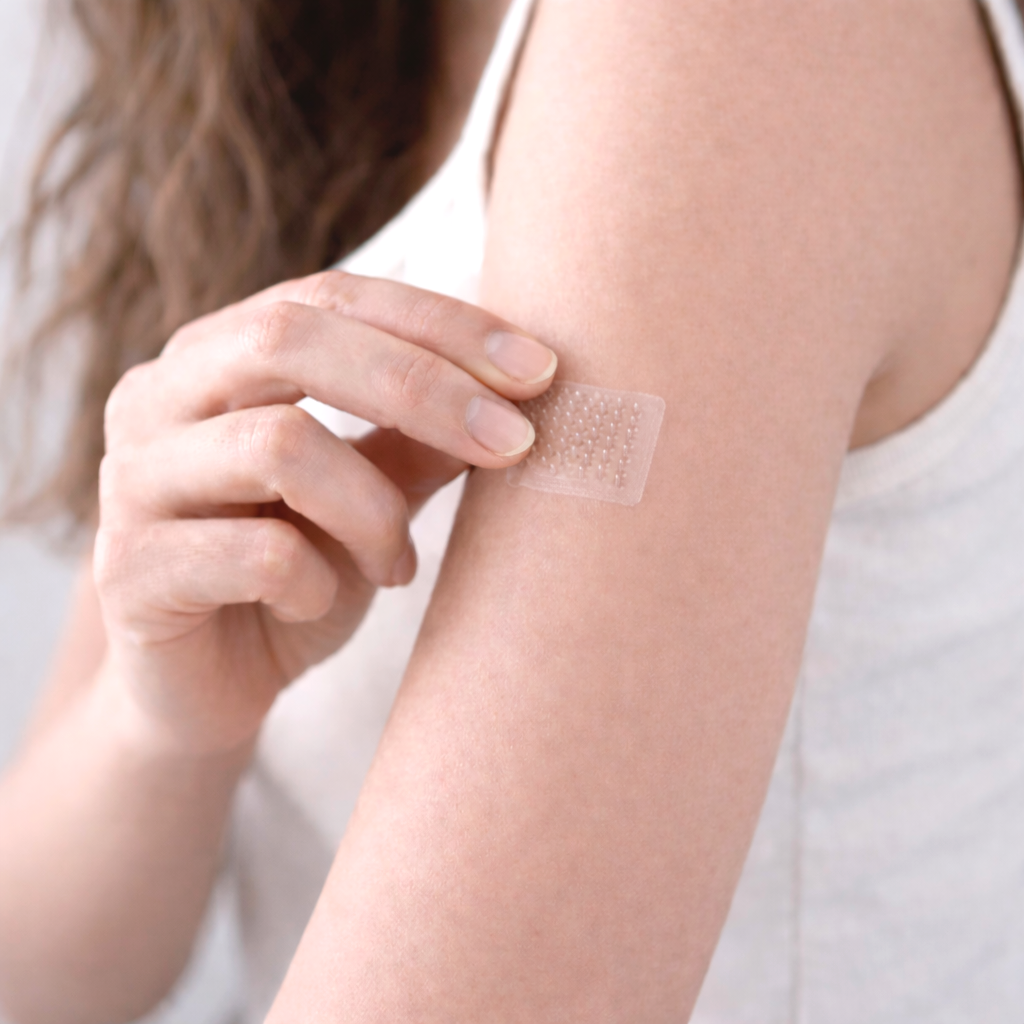
Engineering control in drug delivery
EpiQuil is developing dissolvable microneedle systems to explore approaches to consistent, controlled transdermal delivery of pharmaceutical compounds.
EpiQuil was founded on a simple belief: the way medicines are delivered can shape the treatment experience.
We are dedicated to developing dissolvable microneedle systems intended for transdermal administration. Our development programs focus on addressing practical challenges in medication administration, guided by pharmaceutical quality principles and staged preclinical and clinical evaluation.
Drawing on experience in pharmacy, clinical research and medical technology, we collaborate with leading research groups to advance transdermal delivery systems from preclinical validation through clinical translation.
Our Company
Our Vision
-

Precise
Our R&D program is evaluating microneedle geometry, penetration characteristics, and formulation parameters to characterise performance drivers. Preclinical and ex vivo studies have been used to characterise microneedle–skin interaction and mechanical behavior as part of early proof-of-concept work.
-

Controlled
Our pharmaceutical development investigates dissolution profiles and permeation characteristics in ex vivo models following quality-by-design principles. This includes analytical method development focused on understanding release kinetics and a specifications strategy as the program advances.
-

Age-inclusive
Design considerations include ease of application and user interaction across a broad range of potential users. Human-factors and usability-focused research is being undertaken to inform future development decisions and to support appropriate planning for any subsequent clinical evaluation.
Microneedle Technology
EpiQuil is developing dissolvable microneedle systems intended for transdermal drug-delivery applications under investigation.
Our R&D focuses on microneedle geometry, materials and formulation parameters, with the aim of supporting consistency and usability. Performance is being evaluated in preclinical models, and development is guided by pharmaceutical quality principles.
‘‘Progress in science depends on new techniques, new discoveries, and new ideas, probably in that order.’’
— Sydney Brenner —