The Technology
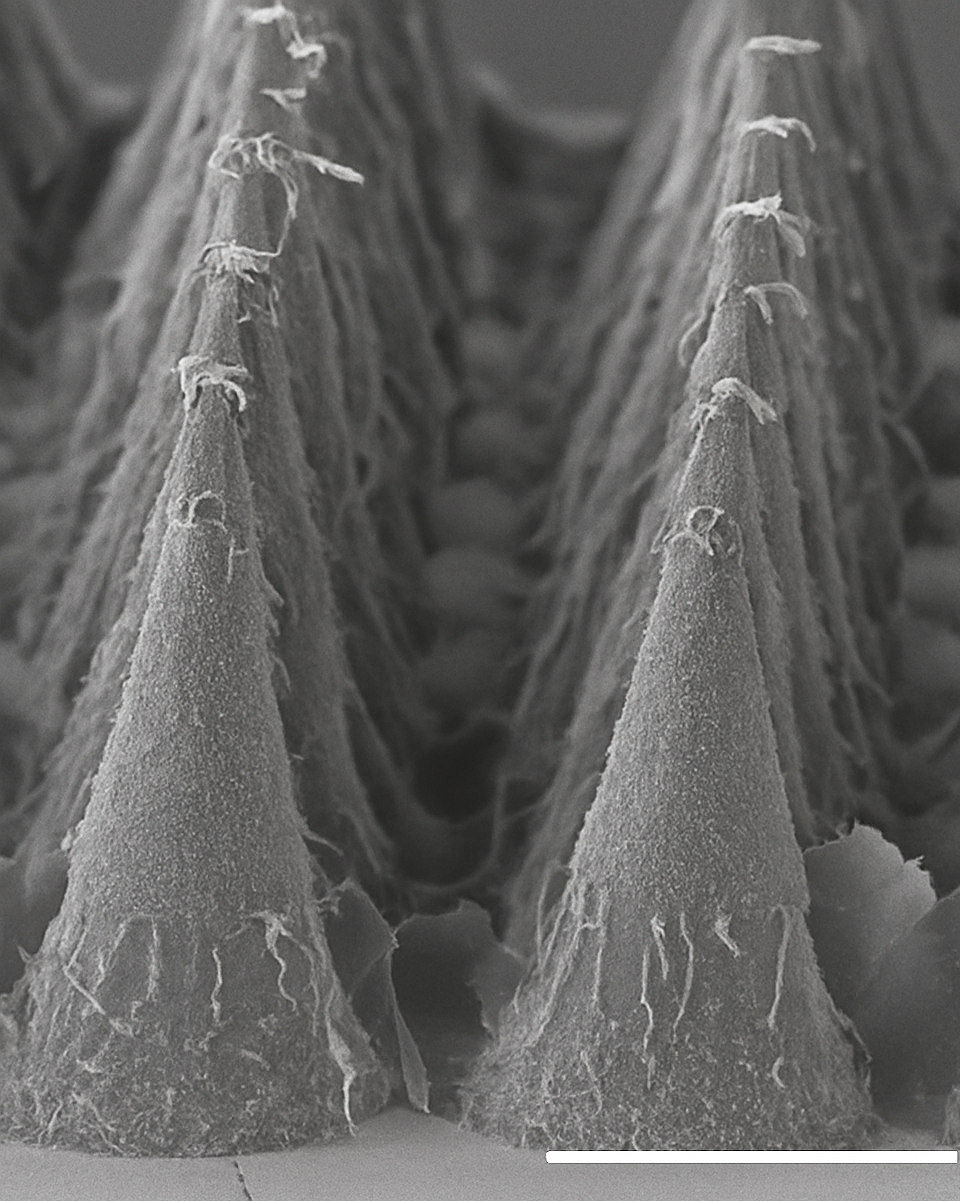
A precise, dissolvable microneedle plateform
Microneedle patches are an emerging class of transdermal delivery systems that use adhesive-backed arrays of microscopic projections intended to interface with the outer layers of the skin.
EpiQuil is developing dissolvable microneedle technologies and conducting structured technical and preclinical work to characterise key parameters relevant to dose delivery, system performance and usability.
Our development focuses on the systematic evaluation of mechanical behaviour at application, dissolution and release kinetics, content uniformity, dose reproducibility and stability under defined storage conditions. This work is supported by analytical testing and quality documentation consistent with pharmaceutical development principles and relevant medical device expectations for drug–device systems.
The objective is to generate robust technical and preclinical evidence that may support progression to future clinical evaluation, while maintaining a consistent emphasis on quality, reproducibility and patient safety throughout the development pathway.
These technologies are under development and are not currently available for supply. They have not been approved by the TGA for therapeutic use. Clinical safety and efficacy have not been established.
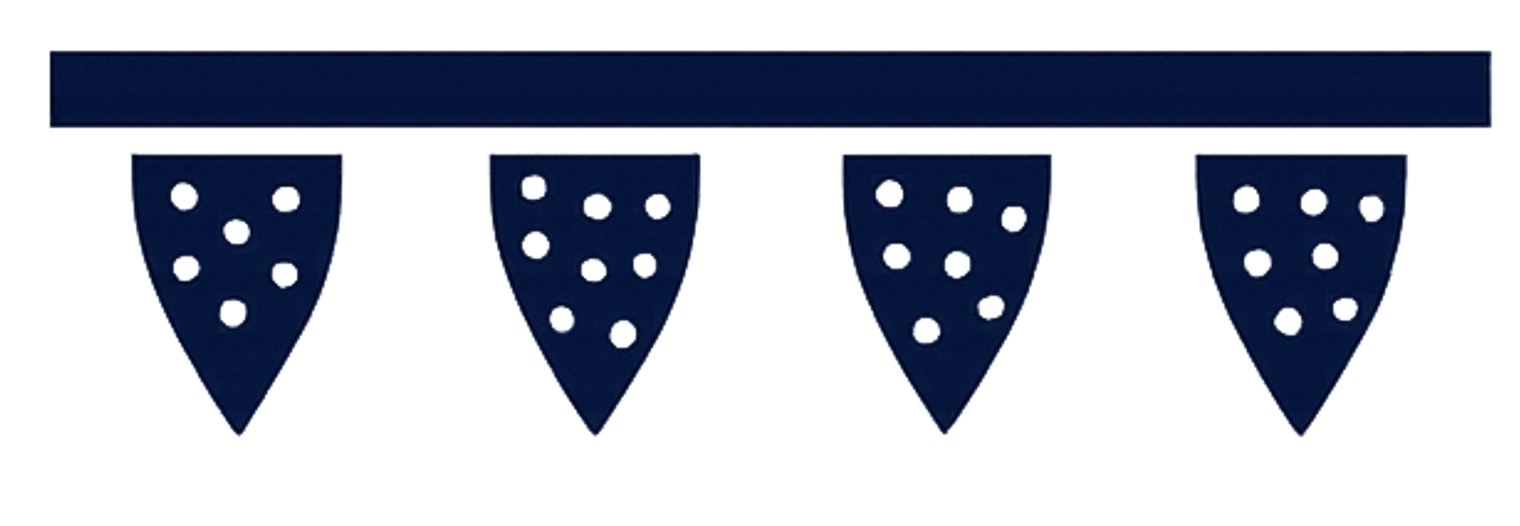
How it works
Our investigational dissolvable microneedle systems are being developed with the aim of supporting reproducible delivery at the skin interface, while reducing residual material.
Press
The patch is applied to the skin, bringing the microneedle array into contact with the outer skin surface.
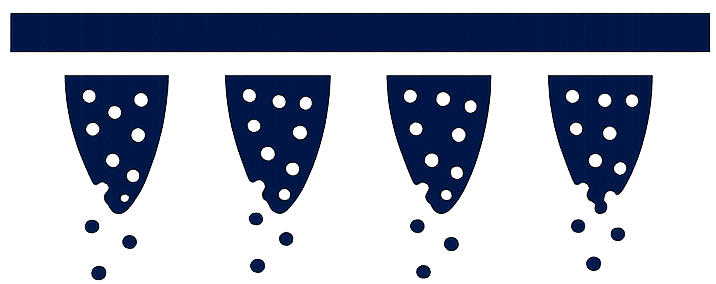
Dissolve
The microneedles are designed to dissolve after application; dissolution behaviour is characterised during development.
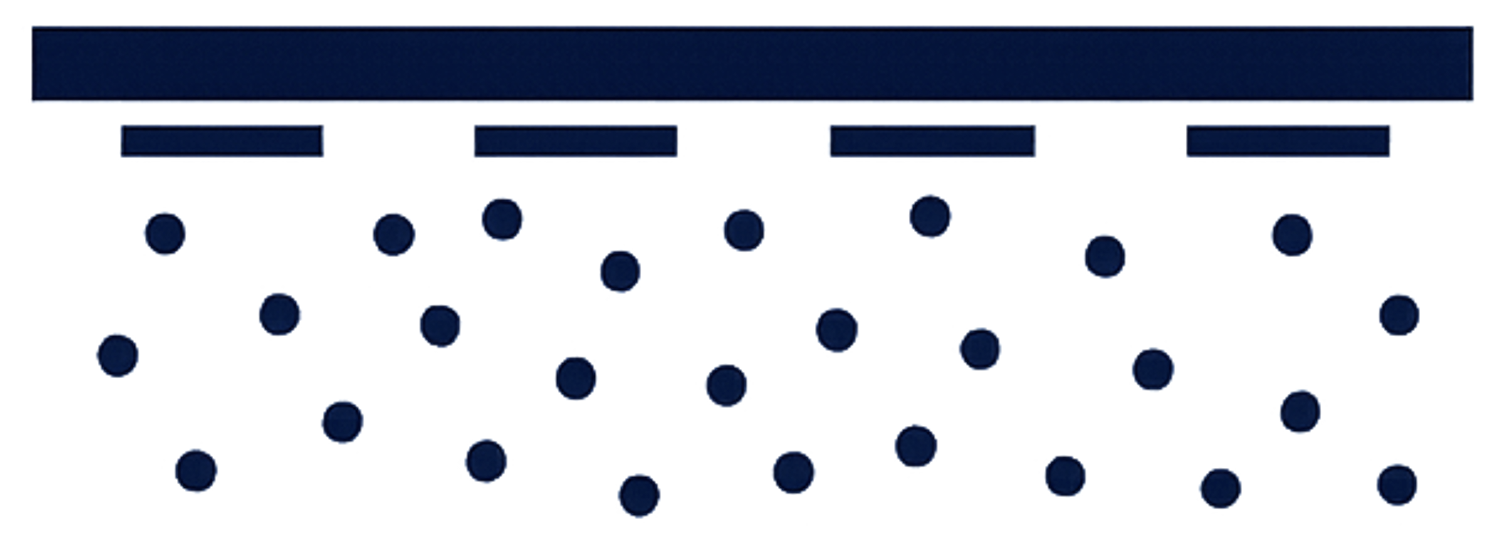
Disperse
As the microneedles dissolve, the active ingredient is intended to be released within the skin.
Schematic representation only. Technology under development, not approved for therapeutic use.
Why it matters
Where innovation meets patient-centered design
Transdermal drug delivery presents well-recognised technical and practical challenges, particularly when consistent administration, usability, and patient acceptability are critical considerations. In clinical practice, factors such as route of administration, tolerability, and access to care settings can influence how therapies are used and experienced.
EpiQuil’s research is focused on exploring dissolvable microneedle technologies as a potential approach to addressing some of these challenges at the level of the skin interface. Development efforts prioritise the characterisation of microneedle–skin interaction, dissolution behaviour, and system reproducibility through preclinical and technical evaluation.
Early-stage programs are designed to generate the data required to assess whether these systems may be suitable for further development as alternative delivery platforms for selected pharmaceutical compounds, subject to appropriate regulatory review and clinical investigation.
All technologies are investigational. Clinical safety and efficacy have not been established, and no products are currently approved for therapeutic use.